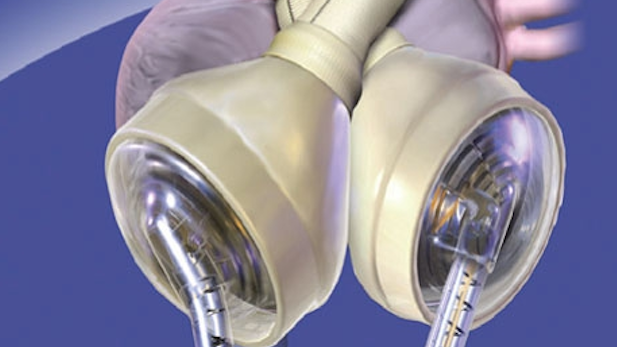
 SynCardia's artificial heart.
SynCardia's artificial heart.Listen:
Tucson-based SynCardia Systems Inc. has received federal approval to test its artificial heart for patients needing it permanently.
Approval for the testing, from the Federal Drug Administration, came last month and was announced by SynCardia Tuesday.
Until now, the SynCardia device has been used on a temporary basis, until patients receive human heart transplants from donors.
Following the approval, SynCardia will test whether its hearts can be used permanently, on people who are ineligible for transplants.
Don Isaacs, vice president of communications at SynCardia, said Tuesday there are a number of reasons, including age or other medical conditions, that make people with heart disease ineligible for transplants.
In the next month, 19 such patients across the U.S. will receive SynCardia hearts for testing. The test group hasn’t yet been chosen.
They will be monitored closely for up to five years, but Isaac said SynCardia will start sending results to the FDA six months after the patients receive their artificial hearts.
The results will help the FDA decide on final approval of the device for permanent use.

By submitting your comments, you hereby give AZPM the right to post your comments and potentially use them in any other form of media operated by this institution.